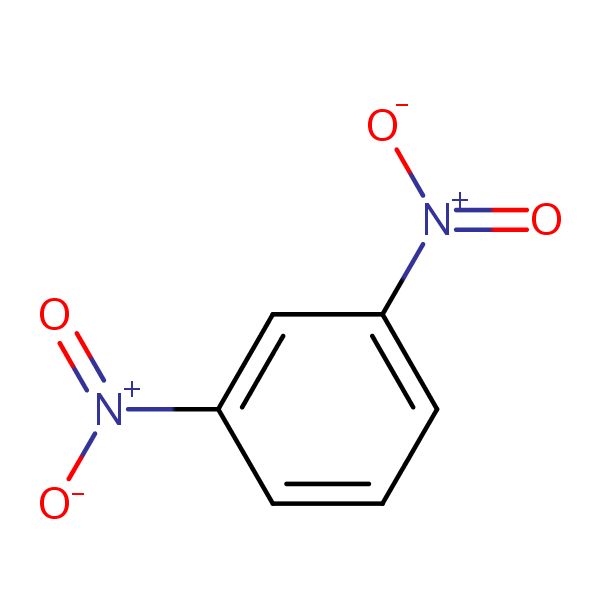
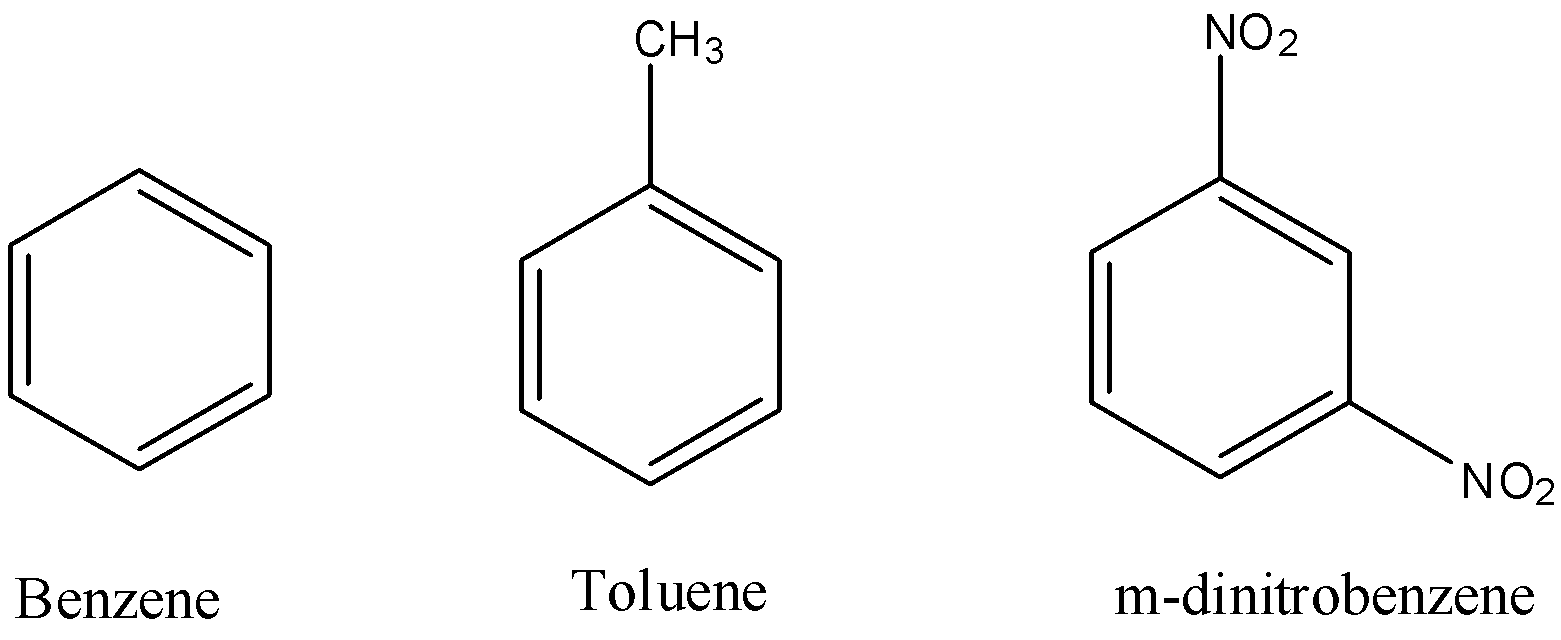
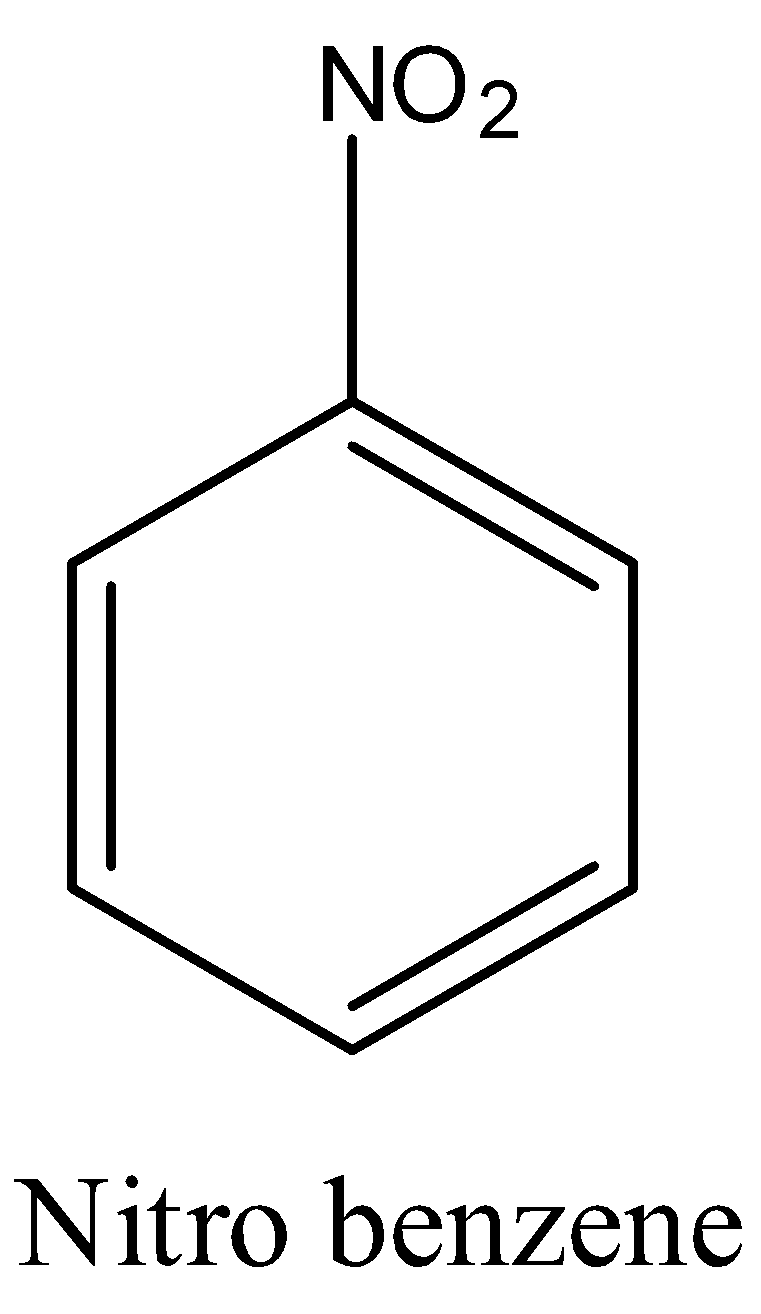
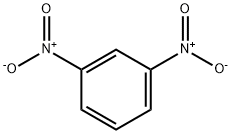
organic chemistry - What is the most accepted mechanism for the reaction of ketones with m-dinitrobenzene? - Chemistry Stack Exchange

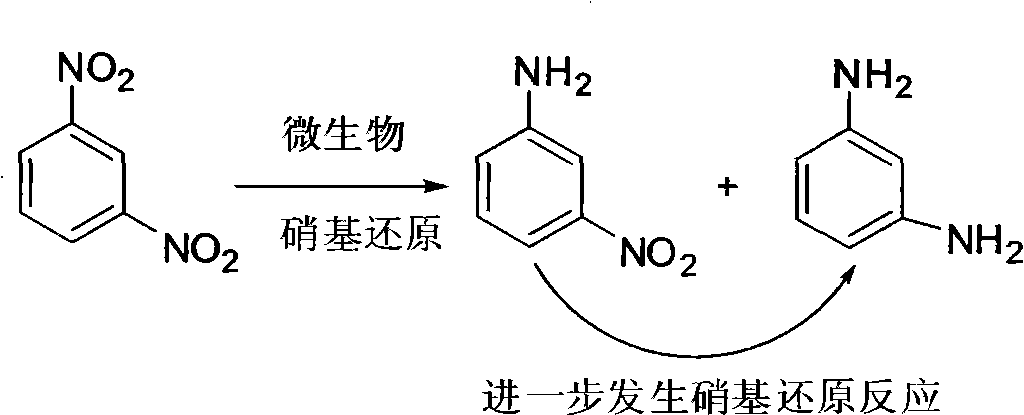
Kinetics and mechanism of the hydrogenation of m-dinitrobenzene to m-phenylenediamine | Reaction Kinetics, Mechanisms and Catalysis

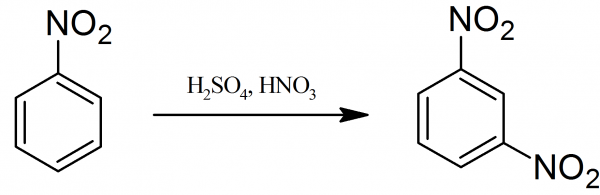
Safe, Green, and Efficient Synthesis of m‐Dinitrobenzene via Two‐Step Nitration in a Continuous‐Flow Microreactor - Zhao - 2023 - ChemistrySelect - Wiley Online Library

100% selective yield of m-nitroaniline by rutile TiO2 and m-phenylenediamine by P25-TiO2 during m-dinitrobenzene photoreduction - ScienceDirect


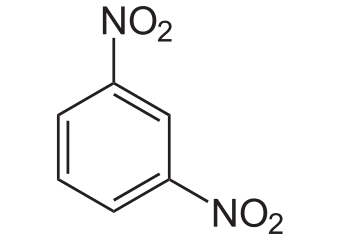

![m-Dinitrobenzene - Optional[UV-VIS] - Spectrum - SpectraBase m-Dinitrobenzene - Optional[UV-VIS] - Spectrum - SpectraBase](https://spectrabase.com/api/spectrum/4DtLkxNWRfO/structure.png?h=300&w=382)
![m-Dinitrobenzene - Optional[MS (GC)] - Spectrum - SpectraBase m-Dinitrobenzene - Optional[MS (GC)] - Spectrum - SpectraBase](https://spectrabase.com/api/spectrum/GR29hrWesE7/structure.png?h=300&w=382)